RGF® first developed its Photohydroionization® (PHI-CELL®) technology over 20 years ago and since then more than four million units have been installed worldwide. Over the years the PHI-Cell evolved into the REME-HALO® and now the widely popular HALO-LED™.
RGF® has licensed its technology to many Fortune 500 companies for use in health care, food processing, military, government, marine, hospitality, residential and commercial applications. In addition, RGF®’s PHI-Cell technology has been specified in the Norovirus and MRSA protection plan of America’s largest restaurant chains, hotel chains, theme parks, cruise lines and public school.
Below you will find a summary of some of the testing and studies performed by third party independent labs and universities. While the research is compelling, RGF® PHI-Cell products are not medical devices and no medical claims are made.
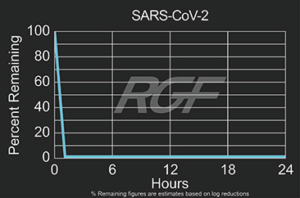
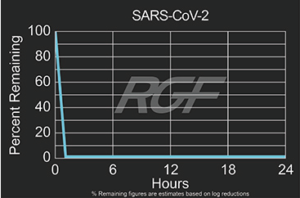
SARS-CoV-2 – Virus – 99+% Airborne Inactivation with REME-HALO®
Testing Summary: 99+% Airborne Inactivation of SARS-CoV-2 (Virus causing COVID-19)
SARS-CoV-2 is an airborne virus that caused the global COVID-19 pandemic. The REME-HALO® was tested at an independent laboratory* for airborne inactivation of SARS-CoV-2 (USA_WA1/2020). Studies were conducted inside a large chamber (1,280 cu. ft.) using a direct air sampling method of the aerosolized virus, resulting in >99% reduction of the virus in the air. RGF’s REME-HALO® reduces the risk assaociated with the SARS-CoV-2 virus in treated indoor air environments. Testing continues on SARS-CoV-2 on various RGF products for both surface and airborne viral reduction.
*Tested by Innovative Bioanalysis, Cypress CA
SARS-CoV-2 – Virus – 99+% Inactivation with REME-HALO®
Testing Summary: 99+% Inactivation of SARS-CoV-2 (Virus causing COVID-19)
SARS-CoV-2 is an airborne virus that caused the global COVID-19 pandemic. The REME-HALO® with PHI was tested at an independent laboratory* for inactivation of SARS-CoV-2 (USA_WA1/2020). Studies were conducted in a large test chamber (8’x8’x20’) on inoculated surfaces and on surfaces exposed to the aerosolized virus resulting in >99% reduction. RGF’s REME-HALO® reduces the risk associated with the SARS-CoV-2 virus in treated indoor air environments. Testing continues on SARS-CoV-2 on various RGF products for both surface and airborne viral reduction.
*Tested by Innovative Bioanalysis, Cypress CA
SARS-CoV-2 – Virus – 99+% Airborne Inactivation with PHI-PKG14
Testing Summary: 99+% Airborne Inactivation of SARS-CoV-2 (Virus causing COVID-19)
SARS-CoV-2 is an airborne virus that caused the global COVID-19 pandemic. The PHI-PKG14 PHI-CELL® was tested at an independent laboratory* for airborne inactivation of SARS-CoV-2 (USA_WA1/2020). Studies were conducted inside a large chamber (1,280 cu. ft.) using a direct air sampling method of the aerosolized virus, resulting in >99% reduction of the virus in the air. RGF’s PHI-PKG14 PHI-CELL® reduces the risk associated with the SARS-CoV-2 virus in treated indoor air environments. Testing continues on SARS-CoV-2 on various RGF® products for both surface and airborne viral reduction.
*Tested by Innovative Bioanalysis, Cypress CA
SARS-CoV-2 – Virus – 99+% Inactivation with PHI-PKG14
Testing Summary: 99+% Inactivation of SARS-CoV-2 (Virus causing COVID-19)
SARS-CoV-2 is an airborne virus that caused the global COVID-19 pandemic. The PHI-PKG14 PHI-CELL® was tested at an independent laboratory* for inactivation of SARS-CoV-2 (USA_WA1/2020). Studies were conducted inside a large chamber (1,280 cu. ft.) on inoculated surfaces and on surfaces exposed to the aerosolized virus resulting in >99% reduction. RGF’s PHI-PKG14 PHI-CELL® reduces the risk associated with the SARS-CoV-2 virus in treated indoor air environments. Testing continues on SARS-CoV-2 on various RGF® products for both surface and airborne viral reduction.
*Tested by Innovative Bioanalysis, Cypress CA
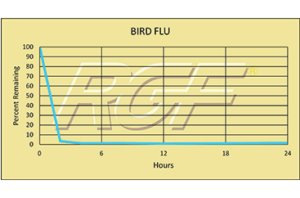
Avian Influenza/ Bird Flu – Virus – 99+% Inactivation
Testing Summary: 99+% Inactivation of Avian Influenza (Bird Flu)
Avian influenza is an infection caused by avian (bird) influenza (flu) viruses. These influenza viruses occur naturally among birds. Wild birds worldwide carry the viruses in their intestines, but usually do not die from them. However, avian influenza is very contagious among birds and can make some domestic birds, including chickens, ducks and turkeys, very sick and kill them. Of the few avian influenza viruses that have crossed the species barrier to infect humans, H5N1 has had the largest number of detected cases of severe disease and death in humans. Of the human cases associated with the H5N1 outbreaks in poultry and wild birds in Asia, Europe, the Near East and Africa, more than half of those people reported infected with the virus died. While there has been some human-to-human spread in H5N1, it has been limited, inefficient and un-sustained.
Source: CDC: Center for Disease Control and Prevention
Tested by Kansas State University Inactivation Rate 99+%
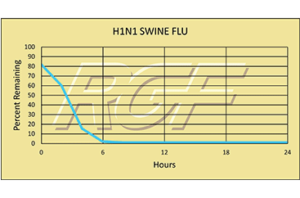
H1N1/ Swine Flu – Virus – 99+% Inactivation
Testing Summary: 99+% Inactivation of H1N1 Swine Flu
The H1N1 flu virus, or swine flu, caused a worldwide pandemic in 2009-2010. It is now considered a seasonal flu, which continues to circulate seasonally worldwide. Spread of the H1N1 virus occurs in the same way that seasonal flu spreads. Flu viruses are spread mainly from person to person through coughing or sneezing by people with influenza. Sometimes people may become infected by touching items – such as a surface or object – with flu viruses on it and then touching their mouth or nose. Kansas State University completed preliminary testing on RGF’s Photohydroionization® (PHI-CELL®) and Reflective Electromagnetic Energy (REME® Cell) technologies with 99+% inactivation of H1N1 Swine Flu on a stainless steel surface.
Tested by Kansas State University Inactivation Rate 99+%
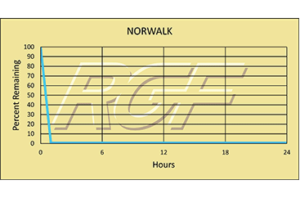
Norovirus/ Norwalk Virus – 99+% Inactivation
Testing Summary: 99+% Inactivation of Norwalk Virus (Norovirus)
Norovirus is a highly contagious virus and as few as 10 viral particles may be sufficient to infect an individual. Infections of the virus can occur by consuming contaminated food or water, by touching contaminated surfaces, or from person-to-person transmission. Norovirus is named after the original strain “Norwalk virus,” which caused an outbreak of gastroenteritis in a school in Norwalk, Ohio in 1968. The most common Norovirus outbreak settings include healthcare facilities, restaurants and catered events, on cruise ships and in schools. 50% of all food-borne outbreaks of gastroenteritis can be attributed to norovirus.
Source: CDC-Centers for Disease Control and PreventionTested by Midwest Research Institute Inactivation Rate 99+%
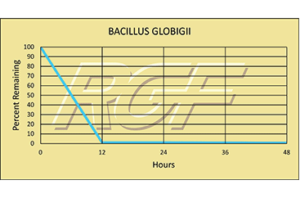
Bacillius Globigii – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Bacillius Globigii
Bacillius Globigii lives in soils around the world and can readily be found in samplings of wind-borne dust particles. It is also known as Bacillus subtilis, its more modern name.
Information Source: CDC CENTER FOR DISEASE CONTROL AND LOS ALAMOS NATIONAL LABORATORY
Tested by Kansas State University Inactivation Rate 99+%
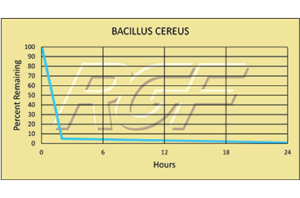
Bacillus Cereus/ B. Cereus – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Bacillus Sp. in 24 hours
Bacillus cereus is a gram-positive, facultatively aerobic spore-former whose cells are large rods and spores do not swell the sporangium. These and other characteristics, including biochemical features, are used to differentiate and confirm the presence of B. cereus. These characteristics are shared with B. cereus var. mycoides, B. thuringiensis and B. anthracis. B. cereus food poisoning is the general description, although two recognized types of illness are caused by two distinct metabolites. All people are believed to be susceptible to B. cereus food poisoning.
Source: U.S. Food and Drug Administration
Tested by Kansas State University Inactivation Rate 99+%
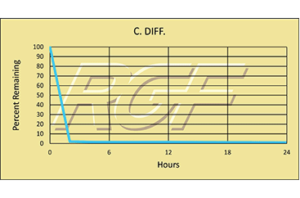
C. Diff./ Clostridium Difficile – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of C-Diff
Clostridium difficile, also known as C-diff or C. difficile, is a gram-positive bacterium that can cause an inflammation known as colitis. It is considered a healthcare-associated infection (HAI), which is an infection that patients get while receiving treatment for medical or surgical conditions. C-Diff infection rates have been on the rise and are becoming more severe and difficult to treat.
Tested by Kansas State University Inactivation Rate 99+%
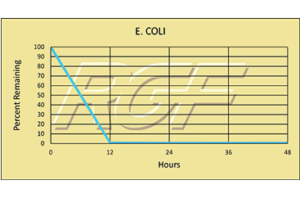
Escherichia Coli (e. coli) – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Escherichia coli
Escherichia coli, usually abbreviated E. coli, are a large and diverse group of bacteria, which are found in in the lower intestines of mammals. Some strains such as enterohaemorrhagic E. coli (EHEC) that are pathogenic can cause severe foodborne illness. Outbreaks are most often linked to raw or undercooked meat products, raw milk, and fecal contamination of vegetables. Source: WHO World Health Organization Tested by Kansas State University Inactivation Rate 99+%
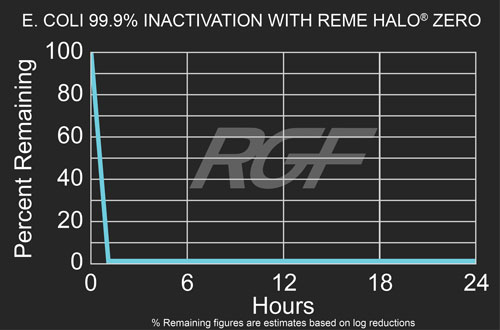
Escherichia Coli (e. coli) – Bacteria – 99.9% Inactivation with REME HALO® Zero

Testing Summary: 99.9% Inactivation of Escherichia Coli (e. coli)
Escherichia Coli (e. coli) is a common intestinal bacterium mostly found in the lower intestine of humans and animals. With respect to the evaluation of air cleaning technologies, e. coli was used to represent common bacterial contamination that may exist in indoor environments. Testing performed in 1,000 cubic foot chamber. The percentage reduction associated with REME HALO® Zero was 99.9% within 60 minutes of exposure. Source: WHO World Health Organization Tested by: Intertek Microbiology Laboratory Columbus, OH
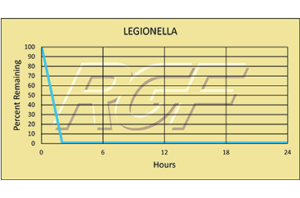
Legionella – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Legionella as tested on PanSaver®
Legionella is a group of pathogenic bacteria that is one of the most frequent causes of waterborne disease in humans. They are found naturally in the environment, and grow best in warm water. One type of pneumonia caused by Legionella is called legionellosis, or commonly known as Legionnaires’ disease. The disease is transmitted when people breathe in a mist or vapor with the bacteria. However, they do not spread from one person to another person.
Information source: CDC Centers for Disease Control
Tested by Kansas State University Inactivation Rate 99+%
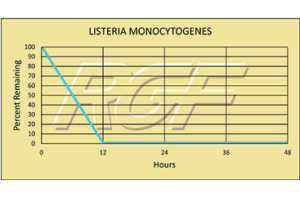
Listeria Monocytogenes – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Listeria
Listeria monocytogenes is a bacterium that causes listeriosis. They are commonly found in soil and water. Animals can carry the bacterium without appearing ill and can contaminate foods of animal origin, such as meats and dairy products. Most human infections follow consumption of contaminated food such as uncooked meats; unpasteurized milk and cheeses, and cooked or processed foods such as ready-to-eat meats. Unlike most bacteria, Listeria is able to grow at refrigerated temperatures. The disease primarily affects older adults, pregnant women, newborns, and adults with weakened immune systems.
Source: CDC Centers for Disease Control
Tested by Kansas State University, Steris Labs, KAG/Eco Labs Inactivation Rate 99+%
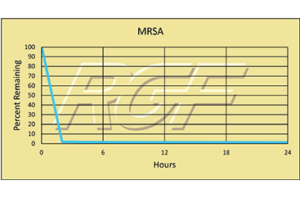
MRSA – Bacteria – 99+% Inactivation

Testing Summary: 99+% inactivation of Methicillin Resistant Staphylococcus Aureus (MRSA)
Methicillin-resistant Staphylococcus aureus (MRSA) is a type of bacterium responsible for difficult to treat infections due to its resistance to certain antibiotics. These antibiotics include methicillin and other more common antibiotics such as oxacillin, penicillin and amoxicillin. Staph infections, including MRSA, occur most frequently among persons in hospitals and healthcare facilities (such as nursing homes and dialysis centers) who have weakened immune systems. In the community, most MRSA infections are skin infections. In medical facilities, MRSA causes life-threatening bloodstream infections, pneumonia and surgical site infections. RGF along with a major hospital participated in a two-year study evaluating PHI technology, which resulted in a 33.4% reduction in infections.
Source: CDC Centers for Disease Control and Prevention
Inactivation Rate 99+% – Tested by Kansas State
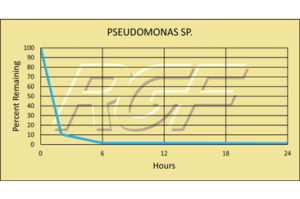
Pseudomonas Sp. – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Pseudomonas sp. in 24 hours
The bacterial genus Pseudomonas includes plant pathogenic bacteria such as P. syringae, the opportunistic human pathogen, P. aeruginosa, the ubiquitous soil bacterium P. putida, and some species that are known to cause spoilage of unpasteurized milk and other dairy products. The Pseudomonads are metabolically diverse, can consequently colonize a wide range of niches, and are generally perceived to be agents of spoilage and degradation
Tested by Kansas State University Inactivation Rate 99+%
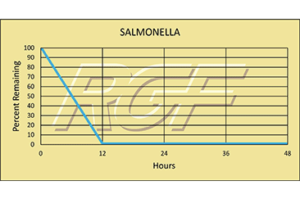
Salmonella – Bacteria – 99+% Inactivation
Testing Summary: 99+% inactivation of Salmonella
Salmonella is the name of a group of bacteria and is one of the most common causes of food poisoning in the United States. Every year one million people are infected, with more than 19,000 hospitalizations and 380 deaths.
Source: Food Safety /CDC Center for Disease Control
Tested by Kansas State University
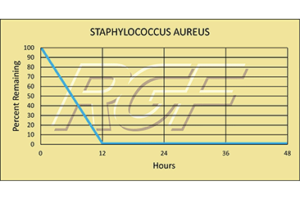
Staph/ Staphylococcus Aureus – Bacteria – 99+% Inactivation
Testing Summary: 99+% Inactivation of Staphylococcus aureus
Staphylococcus aureus, often referred to simply as “staph”, is a bacterium commonly found on the skin and in the nose of people. Person-to-person transmission is the usual form of spread and occurs through contact with secretions from infected skin lesions, nasal discharge or spread via the hands. People infected with staph experience nausea, vomiting, stomach cramps and diarrhea. It is usually mild and most patients recover after one to three days.
Information Source: CDC: CENTER FOR DISEASE CONTROL AND FDA (FOOD AND DRUG ADMINISTRATION)
Tested by Kansas State University Inactivation Rate 99+%
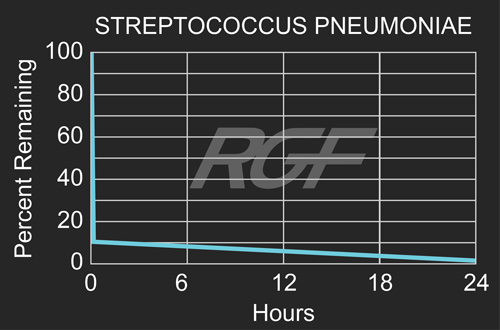
Streptococcus pneumoniae – Bacteria – 90+% Inactivation
Testing Summary: 90+% Inactivation of Streptococcus pneumoniae
According to the Center for Disease Control (“CDC”) Streptococcus pneumoniae is an exclusively human pathogen and is spread from person-to-person by respiratory droplets, meaning that transmission generally occurs during coughing or sneezing to others within six feet of the carrier. Health experts estimate that more than ten million mild infections (throat and skin) like these occur every year.
Source: CDC Centers for Disease Control
Tested by: Kansas State University Average Inactivation Rate Greater than 90+%
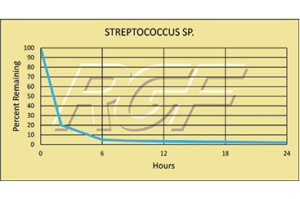
Streptococcus sp/ Strep – Bacteria – 97+% Inactivation

Testing Summary: 97+% Inactivation of Streptococcus sp.
Group A Streptococcal (strep) infections are caused by group A streptococcus, a bacterium responsible for a variety of health problems. Most infections are relatively mild illnesses, such as “strep throat”. These bacteria spread through direct contact with mucus from the nose or throat of people who are sick with an infection, or through contact with infected wounds or sores on the skin.
Source: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
CDC Center for Disease Control
Tested by Kansas State University Inactivation Rate 96+%
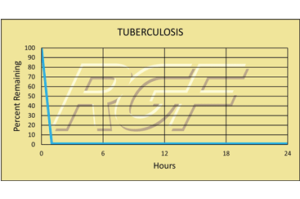
Tuberculosis- Bacteria -99+% Inactivation
Testing Summary: 99+% Inactivation of Tuberculosis
Tuberculosis is caused by the bacterium Mycobacterium tuberculosis. It typically attacks the lungs, but can also affect other parts of the body. It is spread through the air when people with infection cough, sneeze, or otherwise transmit their saliva through the air. Most infections are asymptomatic and latent, but about one in ten latent infections eventually progresses to active disease which, if left untreated, kills more than 50% of those so infected.
Sources: Health and Industry / CDC Center for Disease Control
Tested by Kansas State University Inactivation Rate 99+%
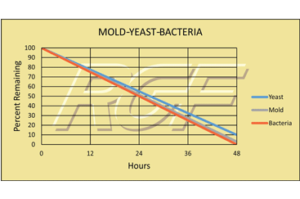
Mold – Yeast – Bacteria

Testing Summary: 97-98% reduction in Mold
Testing Summary: 90+% reduction in Yeast
Testing Summary: 99% reduction in Bacteria
The purpose of these tests was to evaluate the effect RGF’s Advanced Oxidation Technology has on mold, yeast and bacteria (TPC). This test was performed utilizing a standard 2,000 sq. ft. home and 3,000 sq. ft. simulated home.
Tested by California Microbiology Center
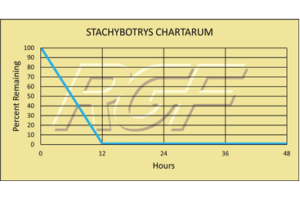
Stachybotrys Chartarum – Fungus/mold – 99+% Inactivation
Testing Summary: 99+% Inactivation of Stachybotrys Chartarum
Stachybotrys is a greenish-black fungus found worldwide that colonizes particularly well in high-cellular material, such as straw, hay, paper, dust, lint, and cellulose containing building materials such as fiber board and gypsum board that become chronically moist or water damaged due to excessive humidity, water leaks, condensation or flooding. Stachybotrys chartaurum grows and produces spores in the temperature range of 36-104F. It is also capable of producing several toxins; however, researchers still know little about the temperature and moisture conditions under which these toxins are produced.
Source: Health and Industry
Tested by Kansas State University Inactivation Rate 99+%
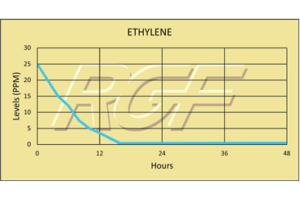
Ethylene/ C2H4 – VOC
Testing Summary: Ethylene levels were reduced 85+% within 12 hours
Ethylene is a naturally occurring small hydrocarbon gas that serves as an aging hormone in plants. Ethylene gas will ripen tomatoes, bananas, pears and a few other fruits postharvest. Premature ripening caused by naturally occurring ethylene gas will negatively influence the fruit’s texture and color, as well as shorten the time cycle for processors to ship to market. By reducing ethylene gas levels, PHI treatment provides the packing house with more time to process and ship before spoilage. A controlled test was performed where high levels of ethylene gas were introduced into a test chamber (25ppm), and when treated with a PHI cell the gas levels were reduced to 3.6 ppm in 12 hours.
Tests were conducted using an ASHRAE compliant HVAC testing system connected to a secondary test chamber.
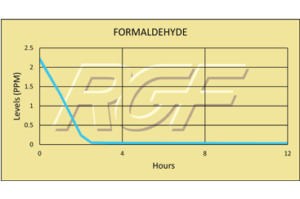
Formaldehyde/ CH2O – VOC
Testing Summary: Formaldehyde levels less than 0.05ppm in 4 hours
Formaldehyde is a colorless, flammable, strong-smelling chemical that is used in building materials and to produce many household products. It is commonly used as an industrial fungicide, germicide and disinfectant. When formaldehyde is present in the air at levels exceeding 0.1 ppm, some people may experience adverse effects including burning sensations in the eyes, nose, and throat, watery eyes, coughing, nausea, as well as skin irritation. The purpose of this test was to evaluate the effect RGF’s Advanced Oxidation Technology has on formaldehyde.
Tests were conducted in a Class II Bio test chamber by Kansas State University
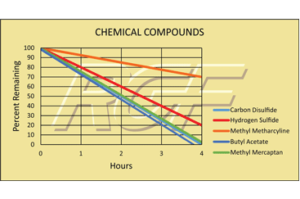
Chemical Compounds – VOCs
Testing Summary: Reduction in 24 hours
Hydrogen Sulfide (Rotten Eggs) – 80% reduction
Methyl Mercaptan (Rotten Cabbage) – 100% reduction
Carbon Disulfide (Vegetable Sulfide) – 30% reduction
Butyl Acetate (Sweet Banana) – 100% reduction
Methyl Metharcyline (Plastic) – 100% reduction
Gas Chromatograph/Mass Spectrometer test performed by Nelap Accredited Lab on airborne chemical compound reduction using RGF’s Advanced Oxidation Technology.
Tested by GC/MS Nelap Accredited Independent Lab
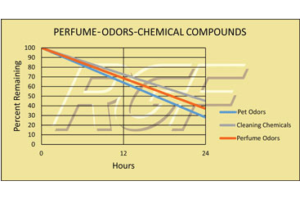
Odors – Perfume, Pet Odors, Cleaning Chemicals

Testing Summary: Cleaning Chemicals – 55% reduction in 24 hours
Testing Summary: Pet Odors – 72% reduction in 24 hours
Testing Summary: Perfume Odors – 63+% reduction in 24 hours
The purpose of this test was to evaluate the effect RGF’s Advanced Oxidation Technology has on cleaning chemicals, pet odors and perfume odors. This test was performed utilizing two 500 cubic foot test chambers and a ten-person odor panel. The qualitative assessments of the ten-person odor panel were then used as a means to determine the odor reduction.
Tested by C&W Engineering (Independent PE Firm)
Smoke Odors

Testing Summary: Smoke Odors – 70% reduction
The purpose of these tests was to evaluate the effectiveness of RGF’s PHI technology against cigarette smoke odors. Tests were performed utilizing two 500 cubic foot test chambers and a ten-person odor panel. The qualitative assessments of the ten-person odor panel were then used as a means to determine the odor reduction.
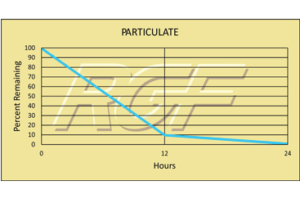
Suspended Particle Reduction – REME®
Testing Summary: Reduction
12 hours ISO Class 4 (10,000 – 0.1um)
24 hours ISO Class 3 (1,000 – 0.1um)
The REME® Cell was evaluated for particulate reduction in a particle test chamber. Particle counts where reduced to ISO Class 4 levels (10,000 – 0.1um) within 12 hours of exposure to the REME® Cell. After 24 hours of treatment, ISO Class 3 levels (1,000 – 0.1um) were achieved. Typical HEPA filtration is effective down to .3um.
Tested by Kansas State University, Performance Analytical Labs
Ozone – EMF-Safety Test
Testing Summary: Passed Federal Safety Standards
RGF Advanced Oxidation Technology devices have been thoroughly tested for ozone / emf – Electro Magnetic Frequency and have passed Federal Safety Standards
Tested by: ETL, TUV to UL standards, CSA, Kansas State University, and other third parties to ensure compliance with federal safety standards.
Note: Many household appliances emit some ozone and emf in safe low levels such as fluorescent lights, motors, computers, copy machines, refrigerators, blenders, electronic air filters, air conditioners, electric fans, microwave ovens, etc.
Electrical Safety Test

Testing Summary: TUV, ETL Approved
RGF’s Advanced Oxidation devices have been thoroughly tested for electrical safety in house, by consultants and certified independent agencies. Results were excellent.
Tested by: TUV to UL standards, ETL, NEI China, RGF Labs, the Japanese Government, Electrical Power Research Institute
Disclaimer: All the above tests were performed on RGF® Advanced Oxidation products with Advanced Oxidation Plasma of less than .02ppm unless noted otherwise. They were conducted by independent accredited labs and university studies. Studies were funded and conducted by RGF®’s major clients to assure third party credibility. RGF products are not medical devices and no medical claims are made.